Abstract
Background: CAR T-cell is a potentially curative therapy for patients with hematologic malignancies but can cause life-threatening toxicities. Data on perceptions of prognosis and psychological distress are lacking.
Methods: We conducted a cross-sectional study of patients receiving CAR T-cell therapy. Prior to hospitalization for CAR T-cell therapy, patients completed assessments of quality of life (QOL) (Functional-Assessment-of-Cancer-Therapy-General), anxiety and depression symptoms (Hospital-Anxiety-and Depression-Scale) and Post-Traumatic Stress Disorder symptoms (Post-Traumatic-Stress-Checklist). Patients also completed the Prognostic-Awareness-Impact-Scale (PAIS), which measures three domains: cognitive understanding of prognosis, emotional coping with prognosis, and adaptive response.
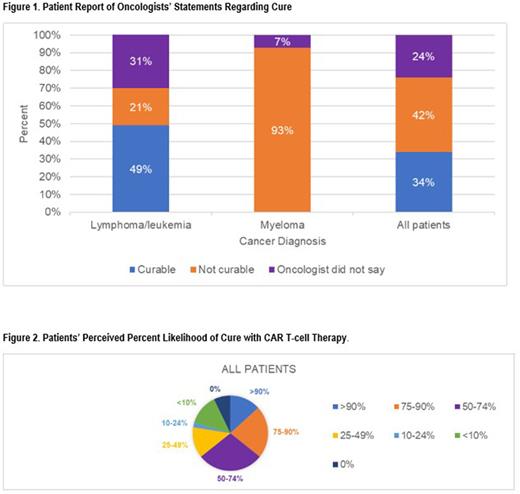
Results: We enrolled 71.8% (102/142) of eligible patients. 34% of patients reported that their oncologist said their cancer is curable and 64% reported there was > 50% chance of cure (Figures 1 and 2). Overall, 26%, 30%, and 21% of patients reported clinically significant depression, anxiety, and PTSD symptoms, respectively. We found no association between patients’ cognitive understanding of prognosis and QOL or mood. Higher emotional coping with prognosis was associated with better QOL (b=0.72; SE=0.10; P=<0.001) and lower depression (b=-0.17; SE=0.02; P=<0.001), anxiety (b=-0.21; SE=0.02; P=<0.001), and PTSD (b=-0.43; SE=0.06; P=<0.001) symptoms. Higher adaptive response was associated with better QOL (b=0.19; SE=0.09; P=0.028) and lower depression (b=-0.05; SE=0.02; P=0.023), anxiety (b=-0.09; SE=0.02; P=<0.001), and PTSD (b=-0.19; SE=0.05; P=<0.001) symptoms.
Conclusions: Patients undergoing CAR T-cell therapy report overly optimistic perception of their prognosis and have high rates of psychological distress. Higher emotional coping with prognosis and adaptive response were associated with better QOL and less psychological distress, underscoring the need to develop interventions to promote coping with this treatment.
Disclosures
Johnson:AstraZeneca: Consultancy. Frigault:JnJ/Legend: Consultancy; Kite/Gilead: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Iovance: Consultancy; Arcellx: Research Funding; Cytoagents: Consultancy; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal